Moderna vs. Pfizer: Why We’re Not Playing Favourites with COVID-19 Vaccines
Everything you need to know about the differences—or lack thereof—between Moderna and Pfizer vaccines, according to experts.
Update: On Sept. 16, Pfizer-BioNTech and Moderna vaccines received full approval from Health Canada as well as new brand names. The Pfizer-BioNTech vaccine is now “Comirnaty,” the Moderna vaccine is now “SpikeVax” and the AstraZeneca vaccine is now called “Vaxzevria.”
Throughout this pandemic, we have been working on COVID-19 vaccine education, communicating the latest evidence and busting harmful myths. Recently, this has involved comparing the Moderna versus Pfizer COVID-19 vaccine.
We’ve received tons of questions on social media and Zoom Q&A calls about the Pfizer COVID-19 vaccine being the vaccine of choice. We’ve also seen hesitation towards—and, at times, outright rejection of—the Moderna vaccine. Concerns range from comparing side effects post-vaccination to speculation about potential supply chain issues with the Moderna vaccine.
To help clear up any confusion, using data from scientific literature, here is what you need to know about the similarities, and the differences between Moderna and Pfizer COVID-19 shots—and the problem with vaccine shopping.
Moderna and Pfizer COVID-19 vaccines are both mRNA vaccines
Both Moderna and Pfizer COVID-19 vaccines use similar messenger RNA (mRNA) technology to generate immunity and protection against the SARS-CoV-2 virus that causes COVID-19. They differ slightly in the chemical composition of lipids, salts and sugars that help keep the mRNA stable. These differences impact storage requirements, but not the effectiveness of the vaccines.
The mRNA in Pfizer and Moderna vaccines contains the genetic recipe for the spike protein found on the surface of the SARS-CoV-2 virus. Both vaccines teach your body to recognize spike proteins, so your immune system is prepared to fight off a SARS-CoV-2 infection quickly enough to avoid severe illness, hospitalization or death.
Moderna and Pfizer provide similarly high levels of protection
Clinical trials showed that Moderna and Pfizer are extremely effective (94.1 percent and 95 percent, respectively) at reducing COVID-19 symptoms. Both vaccines also have similar effectiveness for preventing severe illness and death from COVID-19.
Throughout the global vaccination campaign, both Pfizer and Moderna have worked phenomenally well at protecting people from COVID-19 infections. For example, a large study from U.S. healthcare workers found that the risk of testing positive for SARS-CoV-2 infection was 98.6 percent lower for healthcare workers fully vaccinated with the Moderna vaccine, and 96.8 percent lower for those fully vaccinated with the Pfizer vaccine.
(Related: What You Need to Know About COVID Variants in Canada)
Moderna and Pfizer vaccines are similar in terms of safety
More than 30,000 participants were enrolled into phase 3 trials for the Moderna COVID-19 vaccine and more than 40,000 for the Pfizer COVID-19 vaccine study. The graph below shows the most commonly reported adverse effects (i.e. side effects) for both doses of the Moderna and Pifzer vaccines, such as pain at the injection site and fatigue. This data was reported in the Canadian product monographs, where medication related information is shared for healthcare professionals and the public.
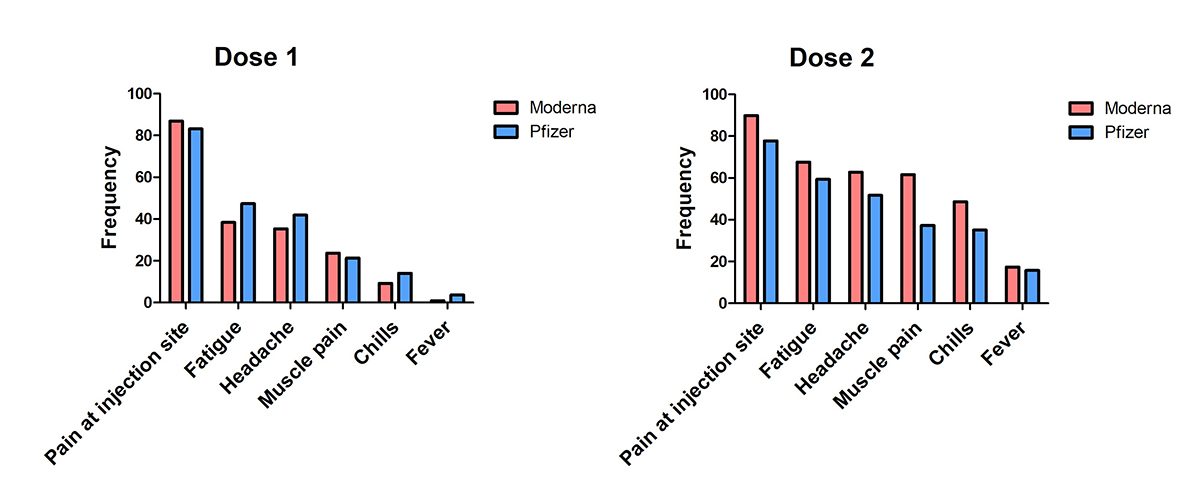
This is not an exhaustive list, but this data shows the similarities between post-vaccine side effects—which also typically resolve within one to three days for both vaccines. We can’t predict how each person will respond to vaccination, but it’s important to keep in mind that these effects are often mild, and a common sign of our immune system being activated. While some people may experience some of these side effects, many people do not.
(Related: 7 Totally Normal COVID-19 Vaccine Side Effects to Be Ready For)
Reported post-vaccine side effects are similar for Moderna and Pfizer vaccines
In Canada, side effects from COVID-19 vaccinations are reported and tracked as part of on-going safety monitoring. Of the over 5 million doses of Moderna vaccine doses administered, only 0.04 percent of those vaccinated reported non-serious side effects, such as headache or fatigue and only 0.003 percent of those vaccinated reported serious side effects, like anaphylactic allergic reactions. Of the over 18 million doses of Pfizer vaccine doses administered, only 0.02 percent of those vaccinated reported non-serious side effects and only 0.005 percent reported serious side effects.
The side effects described above are mostly minor and serious side effects are typically very uncommon. This is in line with the low numbers of side effects being reported as millions of Canadians become immunized.
Both Moderna and Pfizer are increasing supply and availability across Canada
Canada has been ramping up its vaccine supply, including a new shipment of 7.1 million doses of Moderna arriving in June. To get a sense of Canada’s vaccine supply, this government table is updated twice weekly with details on the distribution and allocation of COVID-19 vaccines, including information on the delivery of both of the mRNA vaccines.
In the unlikely event that a vaccine is unavailable for your second dose, the National Advisory Committee on Immunization (NACI) suggests that a different authorized mRNA vaccine can be administered as a second dose. However, there is no reason to assume that a second dose of the Moderna vaccine will not be available at this time for those who received it as a first dose.
(Related: How to Talk to Your Loved Ones About the COVID-19 Vaccines)
What about the delta variant, or mixing with the AstraZeneca vaccine?
Pfizer received some more attention recently as a study from the U.K. found two doses of their vaccine maintains 88 percent effectiveness against the highly contagious delta variant. Studies from the U.K., Spain and Germany have also confirmed the safety and strong immune response of receiving a second dose of Pfizer after a first dose of AstraZeneca. As we wait for data on the Moderna vaccine in these conditions, given their similar composition and performance thus far, Moderna is expected to have similar effectiveness as Pfizer in all aspects.
(Editor’s note: As of June 17, NACI updated its official recommendations stating: “An mRNA vaccine is now preferred as the second dose for individuals who received a first dose of the AstraZeneca/COVISHIELD vaccine, based on emerging evidence of a potentially better immune response from this mixed vaccine schedule and to mitigate the potential risk of VITT associated with viral vector vaccines.”)
The takeaway message
Both the Moderna and Pfizer mRNA COVID-19 vaccines are safe and highly effective at stopping COVID-19 in its tracks.
Pfizer has been used more broadly than Moderna and may benefit from brand recognition; however, the two vaccines are indistinguishable in our eyes. The vaccines work in the same way and have similar side effect profiles. We recognize that side effects would be of particular concern to those who do not have paid sick leave in case the symptoms interfere with their ability to work. Your vaccine administrator or public health unit can advise you on how these symptoms can be managed.
The availability of both vaccines across Canada will continue to ebb and flow with current vaccine distribution, but everyone living in Canada is guaranteed to receive their second dose. Delaying vaccination in hopes of getting a “preferred” brand may put an individual at risk of contracting COVID-19, especially with the rise of the delta variant. This variant of concern is more transmissible and may cause more illness, even after one dose. Based on the data, rather than debating between Pfizer and Moderna, getting the first COVID-19 vaccine available to you as soon as you are eligible is truly the best choice you can make.
Mira Maximos is the Antimicrobial Stewardship Pharmacy Lead at Woodstock Hospital in Ontario, a researcher with Centre of Excellence for Women’s Health and the Knowledge Mobilization pharmacist with Spectrum Mobile Health.
Kimberley Gauthier is a Cell Biology Research fellow in Toronto and volunteer science communicator with COVID-10 Resources Canada.
Krishana Sankar is a Cellular and Molecular Biologist, Science Advisor for Science Up First and Science Communication lead for COVID-19 Resources Canada.
Next, Introducing the Brand New and Not Improved Post-Pandemic Me.




