Advil Cold and Sinus Recall: Here’s What You Need to Know
Health Canada warns that the labelling mixup could spell trouble for some consumers.
Health Canada announced concerns that a labelling error could cause users of Advil Cold and Sinus to mix up the daytime and nighttime medications.
The recall from manufacturer GlaxoSmithKline Consumer Healthcare ULC (GSK) affects two different lots of the over-the-counter pills: one lot of the 18-caplet package and one lot of the 36-caplet package. In the affected packages, the labelling on the back of the blister packs is upside down and misaligned. As a result, the information printed on the foil backing mistakenly identifies the nighttime caplets as daytime caplets, and some daytime caplets are labelled as nighttime caplets.
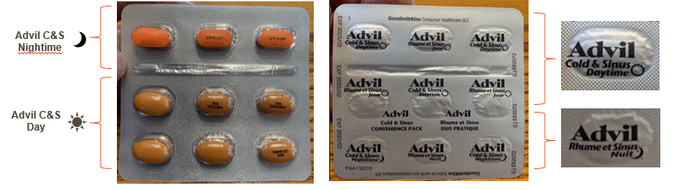
It’s a switch that could spell trouble in particular because the nighttime cold medication is formulated to assist with sleep. While Advil Cold and Sinus daytime is advertised as “non-drowsy,” the nighttime medication contains chlorpheniramine maleate, an antihistamine medicinal ingredient that, as the box indicates, “lets you rest.” Health Canada warns that unknowingly taking drowsy medication could have serious health implications.
“Taking a nighttime caplet when alertness is required may pose potentially serious adverse health consequences, such as when driving motor vehicles or operating heavy machinery. It may also cause potentially serious health consequences for those who have taken other sedatives or tranquilizers, consumed alcohol and the elderly,” warned Health Canada in an October 3 advisory.
The affected products were sold in Canada from July with an expiration date of February 2023. The lots affected are ER2072 and ER2069.
If you have either of the packages of Advil Cold and Sinus being recalled, Health Canada recommends not using the products and either returning them to your local pharmacy for disposal or following local guidelines for the disposal of chemicals and other hazardous waste. Those looking for additional information can contact GSK by calling 1-855-367-7349, or emailing [email protected].
Next: What You Need to Know About the Johnson & Johnson Sunscreen Recall in Canada